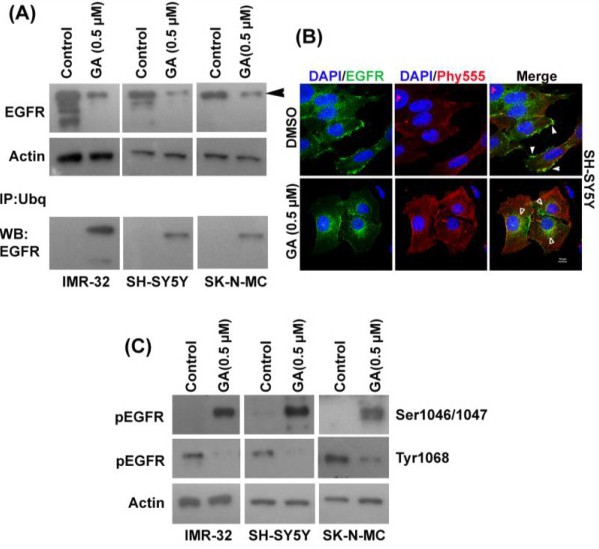
Fig. 5. Gambogic acid treatment induces ubiquitination of the EGF receptor to degrade it. (A) Expression of EGFR was evaluated in IMR-32, SH-SY5Y and SK-N-MC cells after GA administration by Western blot. Actin was used as a loading control. After GA administration to IMR-32, SK-N-MC, and SH-SY5Y neuroblastoma cells, total ubiquitin protein was immunoprecipitated from their cell lysates. EGFR expression was determined by Western blot. (B) SH-SY5Y cells were treated with 0.5 ÁM of GA and incubated for 6 h and fixed. Expression of EGFR was observed using an anti-EGFR antibody. DAPI and Phalloidin Red were used to stain the nuclei and actin filaments, respectively. EGFR expression in the plasma membrane of the cell (white arrows) before GA treatment; post 0.5 ÁM GA treatment at 6 h, EGFR expression is localized in the cytosolic and perinuclear compartments (white-outline arrows). Photographs were obtained by a confocal microscope at 63X magnification. (C) Phosphorylation of EGFR at Ser1046/1047 and Tyr1068 was determined in IMR-32, SH-SY5Y and SK-N-MC cells by Western blot with GA. Actin level was used as the loading control.